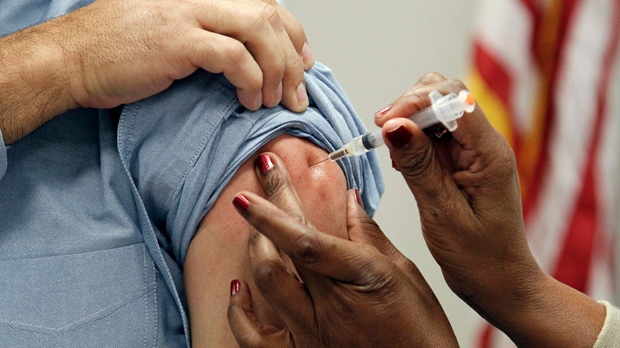
Health Canada is recommending health care professionals across the country hold off on using an Italian flu vaccine that’s been recalled in parts of Europe as officials investigate particles found in vials of the vaccine.
A total of six European countries have banned the vaccine until further testing.
The product was distributed across Canada, and while Alberta health officials here say there haven’t been any adverse effects as a result of the vaccine, they are being advised by the Public Health Agency of Canada, that anyone in possession of the vaccines refrain from using them until after a review is completed.
Italian officials were first to report the problem – after they noticed white particles in vials of flu vaccine manufactured by drug giant Novartis –at a plant in Italy.
Health officials around the world are now testing to determine if the vaccine is safe.
The company maintains the vaccine – which is known as Agriflu in Alberta – is safe.
Health Canada says it has reviewed the safety information and says Canadians recently vaccinated with Agriflu are not at risk.
“We have received about 174,000 doses to date of (the vaccine) in Alberta. Health Canada has done an assessment and there are no reports of “protein aggregation” in Canada. If this were to happen, it would be very visible to the health professional administering the vaccine,” the statement read.
Health officials are required to do a 5-point check before administering Agriflu – making sure each dose is clear of sediment.
If there were particles inside, the dose would not be used.
European health officials expect to have their investigation complete in a few days as the flu season gets underway.
As a precautionary step, Health Canada has asked Novartis to suspend distribution of the vaccines in Canada until a full review of the situation is completed.
Novartis has agreed.
With files from Sonia Sunger