EDMONTON -- Health Canada has ordered the Calgary-based company to stop selling and advertising its COVID-19 rapid test kit. Get the latest here.
Original story: As the number of COVID-19 tests in Alberta reaches 36,000 and climbing, some companies are selling test kits to help businesses flatten the curve when it comes to curbing the spread of the disease.
Calgary-based SBL Testing Technologies specializes in drug and alcohol testing, but says it's now using its technology to detect COVID-19.
The "COVID-19 Rapid Test Kit" has five components and four steps, in which users draw blood from their finger and drop it onto a testing device that promises results in 15 minutes. That's compared to standard testing, which involves a swab inside the nose, and results can take days.
But the device doesn't actually test for the virus itself.
Instead, it detects two antibodies, IgG and IgM, specifically produced to fight COVID-19 five to seven days after symptoms first appear. SBL says the device is more than 97 per cent accurate.
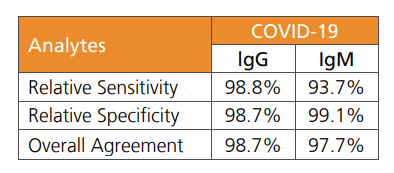
(Source: SBL Testing Technologies)
"This actually tests for the antibodies your body produces once you start to fight off the virus," said SBL managing director Rich Robillard.
SBL says it expects to receive a Class 3 medical device licence from Health Canada soon so it can begin selling kits here, and has already received FDA approval in the U.S.
The kits are only being sold to industry and are not intended for at-home use. They also must be administered by a health care professional.
"Industrial applications, we see everything from doctors and P1s through RNs, [auxiliary] health nurses, paramedics, so depending on who these industrial sites have on looking after their medical, they're the ones who will be performing this service," he said. "This is not for home use, this is not for public sale. This is strictly for health care professionals to provide this service within the workplace."
Some of the company's clients include businesses that operate in manufacturing, mining, oil and gas, energy extraction and forestry.
ORDERS FOR TENS OF THOUSANDS OF KITS STREAM IN
While SBL awaits Health Canada approval, it has already been inundated with orders for the testing kits.
"There's well over 100,000 kits that have been pre-ordered already in Canada," said Robillard. He believes SBL can meet demand in the long-term, though things are "volatile and fluid" day-by-day.
He said the kits will be supplementary to screening practices already used in places like oilsands work camps, which Premier Jason Kenney wants deemed as essential services by the federal government.
"Our list of essential services will be more expansive in energy production than other jurisdictions," Kenney said Wednesday.
Orders of the kit are being taken on a first-come, first-serve basis due to heightened demand.
"For workplace and non-public applications, this qualitative screen can immensely assist in the assessment of individuals potentially showing flu, cold, or COVID-19 symptoms," SBL's website reads.
On Wednesday, CTV News Edmonton's Dan Grummett contacted Health Canada to verify the product was becoming licensed, but a spokesperson said the organization would need time to look into it. Health Canada said on Thursday it would still need more time to gather information.
When asked whether SBL's kits could fall into the hands of the public, Robillard said "there's always that possibility," but the company is doing its best to keep it in the workplace.
Dr. Deena Hinshaw, Alberta's chief medical health officer, was asked Tuesday about another type of at-home kit and had some general views about the emerging industry.
"I know that there are point-of-care tests that have just been licensed in the United States that we are looking into, to see if point-of-care tests might be made available in health care facilities," said Hinshaw.