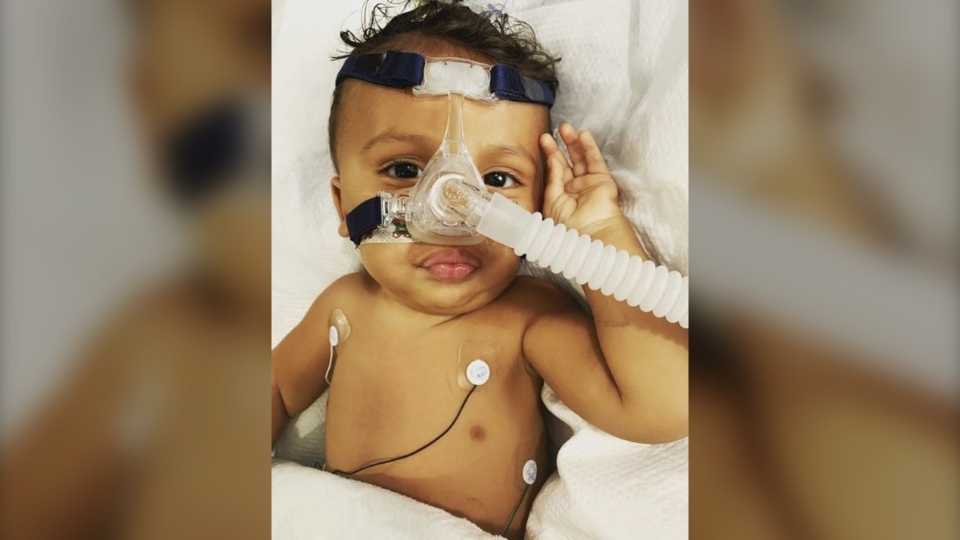
EDMONTON -- An Edmonton family is pleading for Alberta Health compassion funding to get a drug that will significantly improve the quality of life for their 23-month-old son.
Kaysen Martin was diagnosed with Spinal Muscular Atrophy Type 1 (SMA 1), a disease that affects the muscles used for lung support, swallowing, crawling, walking and head control.
The disease affects approximately one in 6,000 babies, and most do not live to see their second birthday, according to the Canadian Organization for Rare Disorders (CORD).
There are limited treatment options; Kaysen currently uses Spinraza, a spinal injection that is administered three times a year for life at an annual cost of $375,000.
The other option is a one-time $2.8 million gene-therapy drug called Zolgensma, which the family says would save AHS thousands of dollars and a lifetime of support for Kaysen.
Zolgensma has FDA approval in the U.S., and the drug company that makes it has applied to Health Canada for approval. However, under Health Canada's Special Access Program, Kayson could get the drug prior to approval if funding becomes available from the government.
"We're appealing to Alberta Health to make an emergency agreement that they will fund this drug ahead of the approval by Health Canada," said Durhane Wong-Reiger, president and CEO of CORD. "They've done it in the past for other conditions, and we're just making an appeal to Alberta Health to do this."
Wong-Reiger says Kaysen's quality of life would be greatly improved on Zolgensma.
"This drug actually replaces the defective gene that he has that causes Spinal Muscular Atrophy," said Wong-Reiger. "We put in a replacement gene, it works just like the SMA gene and Kaysen would in every respect be like a normal child that doesn't have SMA."
Lana Bernardin and Mark Martin, Kaysen's parents, have been lobbying for the drug because Kaysen only qualifies if he's under the age of two. His birthday is on July 17.
“We are exhausted and scared,” said Bernardin in a press release. “But we have to keep fighting."
Bernardin says the family has started a petition to get the drug for Kaysen before his second birthday.
"By the time Health Canada in the provinces approve the funding for this therapy, which we believe inevitably will happen, it's going to be too late for some of these kids because of that two year requirement," said Wong-Reiger.
"We are truly running out of time for Kaysen."
With files from CTV News Edmonton's Bill Fortier
Correction:
A previous version of this article said the Martin family was calling on Alberta Health Services to fund Zolgensma. They are calling on Alberta Health to fund the drug.